Mycoplasma PCR Detection Kit
The e-MycoTM VALiD Mycoplasma PCR Detection Kit is product for the verification of mycoplasma contamination in biological materials.
- • Simple to Use
- – This e-Myco™ VALiD Mycoplasma PCR Detection Kit contains all the components for the PCR reaction.
- – You just add template DNA or samples.
- • Speed – 3 hours
- – Replace traditional 28 days culture testing with the kit within 3 hours
- • Smart – internal control and 8-MOP
- – Internal control system embedded in the product prevents misjudgment that possibly arises from an erroneous PCR test.
- – This kit can eliminate carry-over contamination with 8-MOP activation.
- • Steady – Broad Species Detection
- – You can detect common cell culture-infecting species of mycoplasma and also other various species of mycoplasma.
- • Sensitive and reliable
- – Highly sensitive PCR test for the detection of Mycoplasmas al least 10 CFU/ml
- – Test for validation according to the KFDA testing guidance similar with E.P. 2.6.7 directive.
- – This test is suitable for release testing and in-process control.
- – It can replace culture and indicator cell tests.
SKU: 25239 / 48 Test

299,00€
The e-MycoTM VALiD Mycoplasma PCR Detection Kit is product for the verification of mycoplasma contamination in biological materials.
- • Simple to Use
- – This e-Myco™ VALiD Mycoplasma PCR Detection Kit contains all the components for the PCR reaction.
- – You just add template DNA or samples.
- • Speed – 3 hours
- – Replace traditional 28 days culture testing with the kit within 3 hours
- • Smart – internal control and 8-MOP
- – Internal control system embedded in the product prevents misjudgment that possibly arises from an erroneous PCR test.
- – This kit can eliminate carry-over contamination with 8-MOP activation.
- • Steady – Broad Species Detection
- – You can detect common cell culture-infecting species of mycoplasma and also other various species of mycoplasma.
- • Sensitive and reliable
- – Highly sensitive PCR test for the detection of Mycoplasmas al least 10 CFU/ml
- – Test for validation according to the KFDA testing guidance similar with E.P. 2.6.7 directive.
- – This test is suitable for release testing and in-process control.
- – It can replace culture and indicator cell tests.
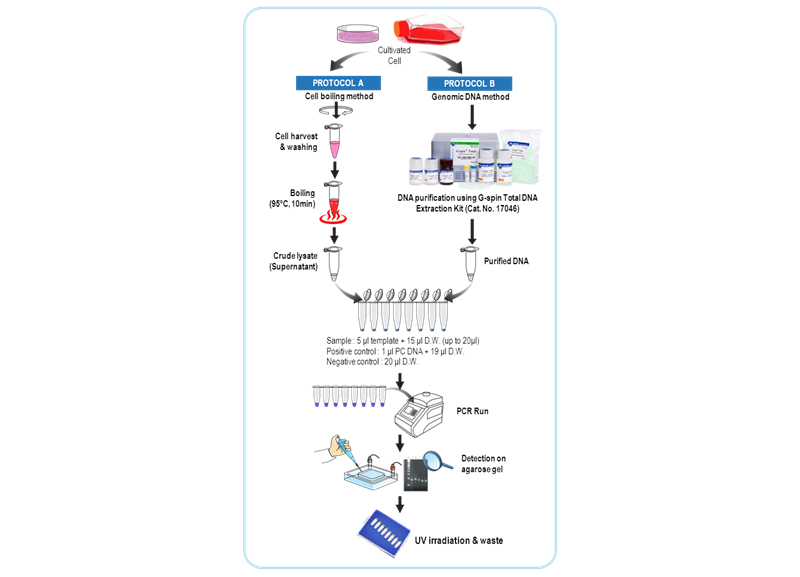
The maintenance of contamination-free cell lines is essential to cell-based research such as biopharmaceutical production, cell therapy and tissue engineering. Mycoplasma is often not visible and does not respond to antibiotics, and therefore it is a major issue that requires monitoring and early detection. Up to 30~85% of cell cultures may be contaminated with mycoplasmas, the main contaminants being the species M. orale, A. laidlawii, M. arginini and M. hyorhinis. Although these mycoplasmas do not usually kill contaminated cells, they are difficult to detect and can cause a variety of effects on cultured cells, including changes in metabolism growth, viability and morphology, thereby altering the phenotypic properties of the host cells. Traditional detection methods use direct culture method to detect contaminating organisms. But the culture-based methods are time-consuming, requiring as much as 28 days, very laborious and difficult to interpret. Thus recently, it is a trend that PCR-based detection method may be adopted to standard protocol replacing direct culture method including E.P. 2.6.7 directive and drug regulating agencies worldwide. The e-Myco™ VALiD Mycoplasma PCR Detection Kit is composed a set of primers those are specific for the highly conserved mycoplasma 16S-rRNA coding region including M. pneumoniae, M. agninini, M. hyorhinis, M. fermentans, M. orale and A. laidlawii. The kit is design to detect the presence of mycoplasma that might contaminate biological materials such as cultured cells. Also, the kit can be performed in 3 hours, can detect sensitively until 10 CFU/ml and includes internal control for verifying a PCR run as well as positive control DNA. The adopted 8-methoxypsoralen (8-MOP) is used to extinguish the template activity of contaminated DNAs (PCR product). 8-MOP is known to intercalate into double-stranded nucleic acids and form a covalent interstrand crosslink after photo-activation by incident light at wavelength 320-400 nm. Each tube of the e-Myco™ VALiD Mycoplasma PCR Detection Kit provides all-in-one system(FastMix technology), which means all components for PCR is already pre-aliquoted in each PCR tube. All you need to do is just to add template and distilled water for PCR.
Applications
The kit is used for the detection of mycoplasma species that are most commonly encountered in cell culture, including M. peumoniae, M. arginini, M. fermentans, M. hyorhinis, M. orale, and Acholeplasma laidlawii. Furthermore, this kit can detect other various species of mycoplasma
Kit Contents
| Contents | 25239 | 25240 |
|---|---|---|
| e-Myco™ VALiD Mycoplasma PCR Premix | 48 Test | 8 Test |
| Control DNA (Recombinant DNA included partial 16S sequence of M. hyorhinis) |
25 μl x 3T | 25 μl x 1T |
| DNase/RNase-free Distilled Water | 1 ml x 1T | 0.2 ml x 1T |






![Nitric Oxide [NO] Plus Detection kit](https://labotaq.com/wp-content/uploads/2019/03/21023-300x300.jpg)















